PURETONE AUDIOMETRY
Audiometers
are used to make quantitative measures of Air Conduction and Bone Conduction Pure
Tone thresholds. AC thresholds assess the entire auditory pathway and are
usually measured using earphones. When sound is delivered by an earphone, the
hearing sensitivity can be assessed in each ear separately. BC thresholds are
measured by placing a vibrator on the skull, with each ear assessed separately,
usually by applying masking noise to the non test ear.
Equipment
AUDIOMETERS
Puretones
are generated within an audiometer. Audiometers have the ability to select
tonal frequency and intensity level and to route tones to the left or right
earphone. All audiometers also have an interrupter switch that presents the stimulus
to the examinee.
The
American National Standards Institute (ANSI) Specification for Audiometers
(ANSI, 2010) describes four types of audiometers: Type 1 having the most features and Type 4 having the fewest features.
Type 1 audiometer
1. Is
a full-featured diagnostic audiometer.
2. A
Type 1 audiometer has earphones,
bone vibrator, loud speakers, masking noise, and other features.
Type 4 audiometer
Is
simply a screening device with earphones, but none of the other special
features. Type 1 (full-featured, diagnostic audiometer) has the ability to
assess puretone AC thresholds for frequencies ranging from 125 to 8,000 Hz and
BC thresholds for frequencies ranging from 250 to 6,000 Hz. If an audiometer has
extended high-frequency capability, air-conduction thresholds can be extended
to 16,000 Hz. Maximum output levels for AC testing are as high as 120 dB HL for
frequencies where hearing thresholds are most sensitive.
Earphones
Earphones
are generally used to test puretone AC thresholds. Supra-aural earphones, ones
in which the cushion rests on the pinna, were the only choice for clinical
audiology. The popularity of supra-aural phones was mainly due to their ease of
calibration and the lack of other types of commercially available earphones. In
the past few years, insert earphones and circumaural earphones have become available
and provide some useful applications for puretone assessment.
Insert earphones
are coupled to the ear by placing aprobe tip, typically a foam plug, into the
ear canal. These earphones have gained popularity in the past few years because
they offer distinct advantages over supra-aural earphones.
Advantages
Insert
earphones yield higher levels of interaural attenuation than supra-aural
earphones.
Interaural
attenuation represents the decibel reduction of a sound as it crosses the head
from the test ear to the nontest ear.
The
average increase in interaural attenuation is roughly 20 dB. This reduces the
need for masking the nontest ear and decreases the number of masking dilemmas,
situations for which thresholds cannot be assessed, because the presentation
level of the masking noise is possibly too high.
Another
important advantage of insert earphones over supra-aural earphones is lower
test–retest variability for thresholds obtained at 6 and 8 kHz; variability for
other frequencies is comparable. Given that thresholds for 6 and 8 kHz are
important for documenting changes in hearing due to noise exposure and for
identifying acoustic tumors, lower variability should increase the diagnostic
precision.
Insert
earphones offer is elimination of collapsed ear canals. Supra-aural earphones
cause the ear canal to narrow or be closed off entirely when the cushion presses
against the pinna, collapsing the ear canal, resulting in false hearing
thresholds, usually in the high frequencies. Because insert earphones keep the
ear canal open, collapsed canals are eliminated.
Insert
earphones is that they can be easily used with infants and toddlers who cannot
or will not tolerate supra-aural earphones.
Insert
earphones is the option of conducting middle-ear testing and otoacoustic emission
testing without changing the earphones; some recently introduced diagnostic
instruments use this approach. Although insert earphones offer a hygienic
advantage over supra-aural earphones, because the foam tips that are placed
into a client’s ear canal are disposable, the replacement cost of those tips is
prohibitive for many applications. In addition to higher costs, insert
earphones also yield errant thresholds in persons with eardrum perforations,
including pressure-equalization tubes for additional
information
about perforations.) Insert earphones also have maximum output levels that are
lower than those produced by supra-aural earphones for some frequencies.
Because of these differences, many diagnostic clinics keep both earphone types
on hand and switch between them depending
on
the application.
Speakers
AC
thresholds can be measured using speakers as the transducer. Thresholds so
obtained are known as sound-field thresholds. Sound-field thresholds are unable
to provide ear-specific sensitivity estimates. In cases of unilateral hearing
losses, the listener’s better ear determines threshold. This limitation and
others dealing with control over stimulus level greatly limit clinical
applications involving sound-field thresholds. Applications for sound-field
thresholds are screening infant hearing or demonstrating to the parents their
child’s hearing ability. Sound-field thresholds may also be desirable for a
person wearing a hearing aid or cochlear implant. In sound-field threshold
measures, the orientation of the listener to the speaker has a large effect on
stimulus level presented at the eardrum. A person’s head and torso as well as
the external ear affect sound levels. Differences in SPL at the eardrum are
substantial for speaker locations at different distances and different angles
relative to the listener. For this reason, sound-field calibration takes into
consideration these factors. A mark is usually made on the ceiling (or floor)
of the room to indicate the location of the listener during testing. Even at
the desired location, stimulus level at the eardrum for some frequencies can
vary as much as 20 dB or more by simply having the listener move his or her
head. Calibration assumes the listener will always be facing the same direction
relative to the sound source. Furniture and other persons in the sound field
also affect the stimulus level at a listener’s eardrum. All of these factors
add to the challenge of obtaining accurate sound-field thresholds. Another
important consideration in sound-field
threshold
measures is the stimulus type. Thresholds corresponding to different
frequencies are desired for plotting an audiogram, but puretones can exhibit
large differences in level at different positions in a testing suite as a
result of standing waves. Standing waves occur when direct sound
from
the speaker interacts with reflections, resulting in regions of cancellation
and summation. Differences in stimulus level due to standing waves are
minimized by using narrowband noise or frequency-modulated (FM) tones as the
stimulus. FM tones, also known as warbled tones, are tones that vary in
frequency over a range that is within a few percent of the nominal frequency. This
variation occurs several times per second. Under earphones, thresholds obtained
with these narrowband stimuli are nearly identical to thresholds obtained with
puretones, with some exceptions in persons with steeply sloping hearing loss
configurations. FM tones and narrowband noise are the preferred stimuli for
sound-field threshold measures.
Bone Vibrators
A
bone vibrator is a transducer that is designed to apply force to the skull when
placed in contact with the head. Puretone BC thresholds are measured with a
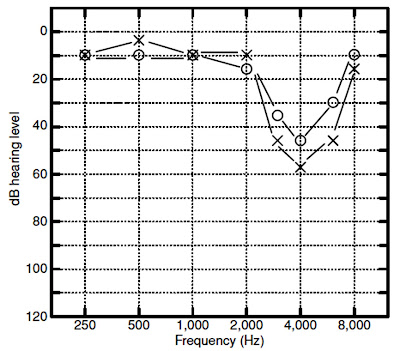
bone vibrator. A separation of 15 Db or more between masked AC and BC
thresholds, with BC thresholds being lower than AC thresholds, is often
evidence of a conductive hearing loss. Other possible explanations for air–bone
gaps and bone–air gaps are equipment miscalibration, test–retest variability,
and individual differences in anatomy that cause thresholds to deviate from the
groupmean data used to derive normative values for relating AC
and
BC thresholds. For threshold measurements bone vibrators are typically placed
behind the pinna on the mastoid process or on the forehead. Placement on the
mastoid process is preferred by 92% of audiologists. Mastoid placement is
preferred mainly because it produces between 8 and 14 dB lower thresholds than
forehead placement for the same power applied to the vibrator, depending on the
frequency (ANSI, 2010). The median difference is 11.5 dB. Given that the
maximum output limits for bone vibrators with mastoid placement are as much as
50 dB lower than that for AC thresholds, forehead placement yields an even
larger difference. The inability to measure BC thresholds for higher levels
means that a comparison of AC and BC thresholds is ambiguous in some cases.
That is,
when
BC thresholds indicate no response at the limits of the equipment and AC
thresholds are poorer
than
the levels where no response was obtained, the audiologist cannot establish
from these thresholds whether the loss is purely sensory/neural or whether it
has a conductive component.