Noise-Induced Hearing Loss
High sound levels can produce both
temporary and permanent hearing losses due to over stimulation and/or mechanical
trauma. A sensorineural hearing loss produced by the
damaging effects of over stimulation by high sound levels, usually over a long
period of time, is called a noise induced
hearing loss. In contrast, the term acoustic trauma usually
refers to the hearing loss produced by extremely intense and impulsive sounds
like explosions or gunshots. They can mechanically traumatize the eardrum,
middle ear, and/or cochlear structures in addition to producing damage by
over stimulation, and often from a single insult.
Almost everybody has experienced temporary hearing
difficulty (often with tinnitus) after being exposed to high sound levels of
one kind or another, such as loud music, construction noise, lawn mowers, subways,
etc. This short-term decrease in hearing sensitivity is sensorineural in nature
and is called a temporary threshold shift (TTS). In general, a TTS can be produced by sound levels
greater than 80 dB sound pressure level (SPL). As the intensity and/or duration
of the offending sound increases, the size of the TTS gets bigger and the time
it takes for recovery gets longer. A permanent
threshold shift (PTS) exists when
the TTS does not recover completely, that is, when hearing sensitivity does not
return to normal. Because PTS could refer to just about any permanent hearing
loss, we generally lengthen the term to noise induced
permanent threshold shift (NIPTS) for
clarity. The nature and severity of a NIPTS is determined by the intensity,
spectrum, duration, and time course of the offending sounds; the overall
duration of the exposures over the years; and the patient’s individual susceptibility
to the effects of noise. In addition, the amount of hearing loss produced by
noise exposure is exacerbated if vibration is also present, and by the use of
potentially ototoxic drugs.
The kinds of anatomical and physiological
abnormalities caused by noise exposure range from the most subtle disruptions
of hair cell metabolic activities and losses of stereocilia rigidity (leading
to “floppy cilia”) to the complete degeneration of the organ of Corti and the
auditory nerve supply. Both outer and inner hair cells are damaged by noise, but
outer hair cells are more susceptible. Some of the abnormalities include
metabolic exhaustion of the hair cells, structural changes and degeneration of
structures within the hair cells, morphological changes of the cilia (so that
they become fused and otherwise distorted), ruptures of cell membranes, and
complete degeneration and loss of hair cells, neural cells, and supporting
cells. Mild metabolic disruptions and floppy cilia can be reversible, and are
thought to be related to TTS. It should be noted in this context that oxidative
stress associated with accumulations of free radicals has been identified as a factor in noise-induced hearing loss Greater amounts of interference
and damage are associated with permanent hearing losses.
Unfortunately, noise exposures capable
of producing temporary hearing loss can
also cause permanent neural
degeneration. Permanent degeneration of the auditory nerve cells even though
the TTS was completely resolved and there was no loss of hair cells. Noise-induced
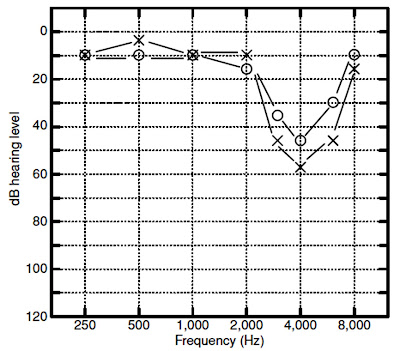
impairments are usually associated with a notch-shaped high-frequency
sensorineural loss that is worst at 4000 Hz although the notch often occurs at
3000 or 6000 Hz as well. The reason for the notch in this region is not
definitively established. One explanation is that this region is most
susceptible to damage due to the biology and mechanics of the cochlea. The
cochlea with a boost in the 2000 to 4000 Hz region because of the resonance characteristics
of the outer and middle ear. Noiseinduced losses tend to be bilateral and more
or less symmetrical; however, there are many exceptions, especially when one
ear has been subjected to more noise than the other. Not all “noise-induced”
audiograms conform to the idealized picture in. Analyses of the progression of
noise-induced hearing losses across many studies have revealed that the general
audiometric pattern of noise-induced hearing loss evolves as noise exposure
continues over the course of many years. The hearing loss typically begins as a
notch at 4000 Hz. As noise exposure continues, the notch widens to include a
wider range of frequencies, but continues to progress most noticeably at 4000
Hz. After perhaps 10 to 15 years of exposure, the progression of the loss at
4000 Hz often slows down, and progression now becomes more apparent at other
frequencies, such as 2000 Hz.
WHY A NOTCH AT
4,000 HZ?
In humans, the frequency of maximum cochlear damage is one-half
to one octave above the frequency of maximum stimulation. This phenomenon has
to do with the angle of curvature of the human cochlea as well as less blood
perfusion in the basal end of the cochlea compared to the apex. The human
external ear (pinna and ear canal) influences the physical properties of sound
outside the head (i.e., in the diffuse field) by resonating at frequencies
between 2,000 and 4,000 Hz, depending on the volume and the length of the ear
canal; for larger adult ears the maximum ear canal resonance, as measured with
a probe microphone, is 2,600 to 3,000 Hz. In children, with shorter ear
canals with a smaller diameter, this ear canal resonance is higher
in frequency. This resonance serves to amplify sound by 15 to 25 dB relative to
the diffuse field (for instance, as measured at the shoulder) at the resonant
frequency. Acousticians and engineers have referred to this resonance as the
transfer function of the open ear (TFOE) or the external ear transfer function
and is known to audiologists as the real ear–unaided response (REUR). When
fitting hearing aids, placement of an earmold results in disruption of this
normal ear canal resonance, resulting in insertion loss. The real ear–aided
response (REAR) must provide amplification to compensate for the insertion
loss, just to get back to the sound level that would arrive at the eardrum
without the earmold or hearing aid in place. For broadband sound, the result of
the TFOE (REUG) is an overall level measured at the eardrum roughly 7 dB higher
than measured at the shoulder. Given that most environmental sound is
relatively broadband, the frequency range of maximum stimulation is roughly
one-half to one octave below 4,000 Hz. This is another reason why the 4,000-Hz
frequency region is the most susceptible to damage.